肌紅蛋白
外觀
| 肌紅蛋白 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||
| |||||||||||||
| 標識 | |||||||||||||
| 代號 | MB; PVALB | ||||||||||||
| 擴展標識 | 遺傳學:160000 鼠基因:96922 同源基因:3916 GeneCards: MB Gene | ||||||||||||
| RNA表達模式 | |||||||||||||
 | |||||||||||||
| 更多表達數據 | |||||||||||||
| 直系同源體 | |||||||||||||
| 物種 | 人類 | 小鼠 | |||||||||||
| Entrez | 4151 | 17189 | |||||||||||
| Ensembl | ENSG00000198125 | ENSMUSG00000018893 | |||||||||||
| UniProt | P02144 | P04247 | |||||||||||
| mRNA序列 | NM_005368.2 | NM_001164047.1 | |||||||||||
| 蛋白序列 | NP_005359.1 | NP_001157519.1 | |||||||||||
| 基因位置 |
Chr 22: 36 – 36.03 Mb |
Chr 15: 76.85 – 76.88 Mb | |||||||||||
| PubMed查詢 | [1] | [2] | |||||||||||
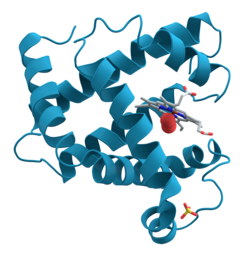
肌紅蛋白(英語:Myoglobin; Mb; MB),又稱肌紅素,是由153個氨基酸環繞中央的血紅素組成的單鏈蛋白質。分子量為16,700道爾頓。其對氧氣的親合力大於血紅蛋白,所以在肌肉組織中有儲存氧氣的功能。因為只需要一點氧分壓便可以使其對氧氣的結合力達到飽和,所以比血紅蛋白更適合儲存氧氣。血紅素對一氧化碳的親和力比氧氣大20,000倍,但是因為肌紅蛋白三級結構上His64(His E7)氨基酸不但可以與氧氣產生氫鍵還可以使一氧化碳偏離原來的結合時的自然狀態,在這一來一往的情形下,使得肌紅蛋白對一氧化碳的親和力只比氧氣高出200倍。由於不具有四級構造,所以不像血紅素一樣,產生協同效應。
若嚴重過度運動,有可能使肌細胞溶解並導致肌紅蛋白進入血液,在腎臟堵住腎小管,引起腎損傷,稱為橫紋肌溶解症。肌細胞溶解還會釋放出大量的鉀,引起高鉀血症。
參考文獻
[編輯]- ^ PDB 1MBO; Takano T. Structure of myoglobin refined at 2.0 Å resolution. II. Structure of deoxymyoglobin from sperm whale. J. Mol. Biol. March 1977, 110 (3): 569–84. PMID 845960. doi:10.1016/S0022-2836(77)80112-5.
延伸閱讀
[編輯]- Collman JP, Boulatov R, Sunderland CJ, Fu L. Functional analogues of cytochrome c oxidase, myoglobin, and hemoglobin. Chemical Reviews. Feb 2004, 104 (2): 561–88. PMID 14871135. doi:10.1021/cr0206059.
- Reeder BJ, Svistunenko DA, Cooper CE, Wilson MT. The radical and redox chemistry of myoglobin and hemoglobin: from in vitro studies to human pathology. Antioxidants & Redox Signaling. Dec 2004, 6 (6): 954–66. PMID 15548893. doi:10.1089/ars.2004.6.954.
- Schlieper G, Kim JH, Molojavyi A, Jacoby C, Laussmann T, Flögel U, Gödecke A, Schrader J. Adaptation of the myoglobin knockout mouse to hypoxic stress. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. Apr 2004, 286 (4): R786–92. PMID 14656764. doi:10.1152/ajpregu.00043.2003.
- Takano T. Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale. Journal of Molecular Biology. Mar 1977, 110 (3): 569–84. PMID 845960. doi:10.1016/S0022-2836(77)80112-5.
- Roy A, Sen S, Chakraborti AS. In vitro nonenzymatic glycation enhances the role of myoglobin as a source of oxidative stress. Free Radical Research. Feb 2004, 38 (2): 139–46. PMID 15104207. doi:10.1080/10715160310001638038.
- Stewart JM, Blakely JA, Karpowicz PA, Kalanxhi E, Thatcher BJ, Martin BM. Unusually weak oxygen binding, physical properties, partial sequence, autoxidation rate and a potential phosphorylation site of beluga whale (Delphinapterus leucas) myoglobin. Comparative Biochemistry and Physiology B. Mar 2004, 137 (3): 401–12. PMID 15050527. doi:10.1016/j.cbpc.2004.01.007.
- Wu G, Wainwright LM, Poole RK. Microbial globins. Advances in Microbial Physiology 47. 2003: 255–310. ISBN 9780120277476. PMID 14560666. doi:10.1016/S0065-2911(03)47005-7.
- Mirceta S, Signore AV, Burns JM, Cossins AR, Campbell KL, Berenbrink M. Evolution of mammalian diving capacity traced by myoglobin net surface charge. Science. Jun 2013, 340 (6138): 1234192. PMID 23766330. doi:10.1126/science.1234192.. Also see Proteopedia article about this finding (頁面存檔備份,存於互聯網檔案館)
外部參考
[編輯]- OMIM 160000 human genetics
- The Myoglobin Protein (頁面存檔備份,存於互聯網檔案館)
- RCSB PDB featured molecule
- Which Cut Is Older? (It's a Trick Question) (頁面存檔備份,存於互聯網檔案館) New York Times, February 21, 2006 article regarding meat industry use of carbon monoxide to keep meat looking red.
- Stores React to Meat Reports (頁面存檔備份,存於互聯網檔案館) New York Times, March 1, 2006 article on the use of carbon monoxide to make meat appear fresh.
- PDB中UniProt可用的所有結構資訊之概述:P02144 (Human Myoglobin) 在PDBe-KB。
