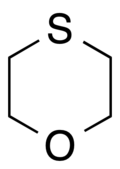
1-氧雜-4-硫雜環己烷
| 1-氧雜-4-硫雜環己烷 | |
|---|---|
| |
| IUPAC名 1,4-Oxathiane | |
| 別名 | 1,4-Thioxane; p-Thioxane; 1-Oxa-4-thiacyclohexane |
| 識別 | |
| CAS號 | 15980-15-1 |
| 性質 | |
| 化學式 | C4H8OS |
| 摩爾質量 | 104.17 g·mol−1 |
| 熔點 | 30 °C(303 K)[1] |
| 沸點 | 147—149 °C(420—422 K)[2] |
| 危險性 | |
GHS危險性符號 
| |
| GHS提示詞 | Warning |
| H-術語 | H226, H315, H319, H335 |
| P-術語 | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P302+352, P303+361+353, P304+340, P305+351+338 |
| 致死量或濃度: | |
LD50(中位劑量)
|
2830 mg/kg oral rat |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
1-氧雜-4-硫雜環己烷是一種有機化合物,化學式為C4H8OS。它可由2,2'-二氯乙醚(或2,2'-二溴乙醚)和硫氫化鉀反應得到,[3]或由硫代二甘醇和丁基三氯化錫反應製得。[4]在催化下,它可以被過氧化氫氧化為亞碸或碸。[5]
參考文獻[編輯]
- ^ James Ranald Alexander, Hamilton McCombie. CCLIX.—The reactions of divinyl sulphide, sulphoxide, and sulphone. J. Chem. Soc. 1931, 0 (0): 1913–1918 [2022-05-30]. ISSN 0368-1769. doi:10.1039/JR9310001913 (英語).
- ^ Frederick Richter, Frederick B. Augustine, Emil Koft, E. Emmet Reid. The Condensation of 2-Hydroxyethyl Sulfides with Alcohols and Phenols 1. Journal of the American Chemical Society. 1952-08, 74 (16): 4076–4079 [2022-05-30]. ISSN 0002-7863. doi:10.1021/ja01136a031. (原始內容存檔於2022-05-30) (英語).
- ^ Movsumzade, M. M.; Gurbanov, P. A.; Askerov, N. D.; Khodzhaev, G. Kh. Reaction of β,β'-dihalodialkyl ethers with potassium hydrosulfide(俄文). Azerbaidzhanskii Khimicheskii Zhurnal, 1979. 6: 86-90. ISSN 0005-2531.
- ^ Giuseppe Tagliavini, Daniele Marton, Donatella Furlani. Organotins as etherfication catalysts. 11. catalytic conversion of alcohols to open-chain and cyclic ethers by organotin trichlorides. Tetrahedron. 1989-01, 45 (4): 1187–1196 [2022-05-30]. doi:10.1016/0040-4020(89)80027-4. (原始內容存檔於2022-07-09) (英語).
- ^ Elena Badetti, Alessandro Bonetto, Francesco Romano, Luciano Marchiò, Cristiano Zonta, Giulia Licini. Synthesis, Characterization and Catalytic Activity of a Tungsten(VI) Amino Triphenolate Complex. Catalysis Letters. 2017-09, 147 (9): 2313–2318 [2022-05-30]. ISSN 1011-372X. doi:10.1007/s10562-017-2144-z (英語).
